The Atlantic cod, Gadus morhua, Fig. 1, is one of the principle ground fish of the northwest Atlantic. Due to its abundance and economic importance to our northeast, it is a target of a great deal of interest for its own sake as well as a model for similar situations. Stresses from exploitation on the northwest Atlantic ground fish populations have had dramatic effects on the age at maturity of several fish species, including that of the Georges Bank Atlantic cod stock (Trippel, 1995). During the 1986 to 1993 period an 18% decline in the median age at maturity of Atlantic cod was observed. These changes in age at maturity raise several questions that need addressing. Though the fish mature at a younger age, in the sense that they are producing eggs, younger fish produce fewer and smaller eggs (Kjesbu, 1989) and hatchability of eggs is usually positively correlated with size of egg and thus age of the female. Population consequences might be detrimental. Many, but not all, of the commercially important fish stocks exhibit the early maturity phenomenon.
 |
| Fig. 1. Atlantic cod, Gadus morhua, adult, illustrated from the Woods Hole Oceanographic Institute WWW site http://www.wh.whoi.edu/library/sos94/spsyn/pg/cod.html |
The current ban on fishing Georges Bank is an attempt to allow the cod and haddock stocks to recover the recent decline documented by Trippel (1995). More tools are needed to evaluate the state of recovery in order to decide when the ban could or should be lifted.
The decline in median age at maturity has been ascribed to several causes related to harvesting of the larger adults: a physiological compensatory response to a thinning population; a more serious genetic selection for survival of early maturation; or a combination of both (Trippel, 1995). The recovery kinetics from the two extreme alternatives would be very different. Methods are needed to quantitatively evaluate the quality of eggs from season to season and to relate that quality to various measurable traits of the originating female including body size, ovarian size and maturation state, serum estradiol titer, serum vitellogenin titer, and otolith age determination. Additional techniques need to be developed to evaluate the phenomenon of early maturity in order to devise rational strategies for starting and ending moratoria restricting harvesting and for establishing regulations on what size and age of fish to declare legal. If early maturing fish are producing perfectly viable eggs, there is less reason to prevent their harvesting. However, if earlier maturing fish are markedly less fertile, continued fishing may be leading to a downward population spiral that may lead to total collapse of the stock. Methods to measure and evaluate this phenomenon are needed.
A major measure of maturity of the female fish is the livers ability to synthesize and secrete Vitellogenin (Vg), the precursor of Lipovitellin (Lv) and phosvitin (Pv), the major protein stores of the ovulated egg. This Vg is synthesized under the direction of the steroid hormone estradiol, secreted into the serum and taken up by adsorptive endocytosis into the growing follicles of the ovary. In the ovary the Vg is processed into Pv and Lv. Vg and Lv have similar immunologic properties and usually the same polyclonal antiserum serves to quantify both. The Pv is a highly phosphorylated peptide which does not stain well in analytical gels and is poorly immunogenic. As a result, Pv is usually ignored in any analysis of the egg's stored yolk, or assumed to be equimolar with its cleavage partner Lv. Serum titer of Vg may be low if the ovarian follicles are very actively removing it from the serum. Likewise Vg could be high in an early maturing fish whose liver is actively producing Vg but whose ovaries are not efficiently removing the secreted Vg into developing follicles. This phenomenon is created artificially by investigators who inject estradiol into immature fish to generate high titers of Vg in the serum (Silversand et al., 1993; Yao and Crim, 1996). We need to know the signs of early maturation in cod better. Does it include a high serum or mucus Vg component? Can we easily recognize, using a mucus or serum test (Kishida et al., 1992), the syndrome of early ovarian maturation? Is early maturation related to a decrease in fecundity for the stock in general? Thus, we need to investigate the relationship of Vg serum and mucus titer to the age and maturation of the female gonads and production of viable eggs.
The Vg of Gadus morhua has been purified from serum of estradiol treated immature cod males or females (Silversand et al., 1993; Yao and Crim, 1996) and some of its properties (amino acid content, lipid content, phosphorous content, apparent Mr , instability) have been determined. Fish Vgs and Lvs have been known to be difficult to purify due to the substantial proteolytic nicking of its constituent peptides. This heterogeneity of the molecule is generated by proteolysis occurring naturally and during the isolation process. It is also further demonstrated that estradiol induced Atlantic cod Vg is particularly vulnerable to proteolysis during isolation and during incubation after extended storage at -20C in 50% glycerol (Silversand et al., 1993). In addition the serum of estradiol injected fish, including Gadus morhua, contain small peptides, not derived from Vg, but which are vitelline envelope components (Hyllner et al., 1991; Hyllner and Haux, 1992; Oppen-Berntsen et al., 1992). These peptides are induced by injected estradiol in either male or female. Thus the strategy for producing a Vg-specific antiserum which uses male serum in an adsorption to clean up non-female-specific antibodies from a polyclonal anti-Vg-serum is not acceptable. We have therefore adopted an Atlantic-cod-Lv purification strategy to produce both a monospecific antiserum and a stable calibration standard. This is based partially on our success with purifying winter flounder Lv and our discovery of a new purification principle, fractional denaturation (Hartling, Pereira and Kunkel, 1997) which combined with gel permeation produces a highly purified and stable Lv antigen for immunization and use as a standard.
The reason that fish Vg is observed to be degraded and fragile
on purification may be due to the artificial manipulation of the donor
fish with injected estradiol over a period of weeks, (Silversand et al.,
1993; Yao & Crim, 1996). Under estradiol treatment, the otherwise
immature fish accumulate an abnormally high content of Vg in their
serum since, lacking vitellogenic follicles, Vg has no normal exit
from the serum. The mucus may be an excretory vehicle in this respect.
This burden of extra protein in the bloodstream certainly leads over the
weeks to physiological responses of the fish to clear or degrade the excessive
amounts of Vg. Indeed, we have observed the 'processing' of Vg peptides,
which normally occurs in the oocyte, to occur in the serum of ovariectomized
insect females (Wojchowski et al., 1986). In Gadus morhua
injected with estradiol small peptides derived from Vg accumulate
in the serum by the time, weeks after the estradiol injection, of harvesting
of the Vg laden serum (Silversand et al., 1993). A protease inhibitor,
aprotinin, is injected into fish a half hour prior to projected bleeding
in order to inhibit proteolytic cleaving of Vg during the bleeding
process. But, this cannot eliminate the cleavage and modifications that
have already occurred during the weeks of estradiol treatment and abnormally
high accumulation of Vg in the serum. I am much more confident in
the integrity of Lv stored in newly ovulated or stripped eggs from
normal adult female fish. Lv is a normally stored protein while
Vg usually has a short life in the serum before it is normally cleared
by endocytosis. We have found that Winter flounder Lv is remarkably
stable (Hartling et al., 1997) and suspect that this
property will extend to Lv of other fish. The fact that Lv
differs from Vg by missing its Pv peptide is inconsequential
since the Pv peptide is known to be a poor antigen. Thus the majority of
the immunologic determinants of Vg are carried on the Lv
peptide.
It is proposed to develop an immunologic assay for Atlantic cod vitellogenin
(Vg) and the derived lipovitellin (Lv) in order to establish
an accurate method for aiding the determination of spawning status, egg
quality and egg viability. This antiserum would be used to measure the
Vg titer in female serum and epidermal mucus and the Lv content
of ovulated eggs as well as pelagic larvae. The Vg serum and mucus
titer (Kishida et al., 1992) of individual females will be correlated with
the maternal age, size and a histological measure of the state of maturity
of her ovary. Particular emphasis will be placed on studying where the
Georges Bank stocks fit in the 35-65 cm 'normal' age/size of females undergoing
ovarian maturation among different cod stocks (discussed in Morrison,
1987). With these measures it will be possible to assess the reproductive
maturity of the female and the health and viability of her eggs and potential
developing embryos (Morrison, 1995). The type
of Vg/Lv assay proposed is an enzyme linked immunosorbent assay (ELISA).
This ELISA was chosen for its sensitivity and ability to be automated plus
its relatively high environmental friendliness when compare to radio-immune
assays (Yao and Crim, 1996) which generate substantial low level liquid
and solid radioactive waste.
Purification of Lv (or Vg).
The starting material for Vg is vitellogenic female serum or estradiol injected males or immature females. This source will be a last resort for production of a homogeneous antigen and immunologic standard since it has already been demonstrated to vary substantially in degradation when it accumulates in the blood of estradiol injected immature Atlantic cod (Silversand, 1993). This is a reasonable last resort since spent males and females could be injected at any time of the off season to produce the high titer Vg in captive cod. This Vg has been successfully purified and used to produce an antiserum to Atlantic cod Vg (Silversand et al, 1993). However, I believe an anti-Lv serum would be superior in that its production is based on a (potentially) more stable antigen and an assay based on anti-Lv would be inherently more able to be reproduced and calibrated year to year. The starting material for Lv purification is newly spawned or stripped unfertilized eggs. Vg and Lv are very large proteins with apparent Mr 485,000 for Atlantic cod Vg (Yao and Crim, 1996). This large size is relatively unique among cellular and serum proteins and has been used effectively in the past to purify Lvs of other species. An initial gel permeation chromatography on BioGel A1.5 of serum or egg extract should produce a major peak of protein eluting with maximal resolution in the middle of the column effluent, fig. 2. If more resolution is needed we have the capacity to recycle the effluent through the 1 meter column to achieve a doubling or tripling of resolution. This is a preparative (as well as analytical) technique which produces enough material to be used as immunizing antigen as well as the more substantial amounts of protein that are needed to serve as calibration standards in each assay that is performed.
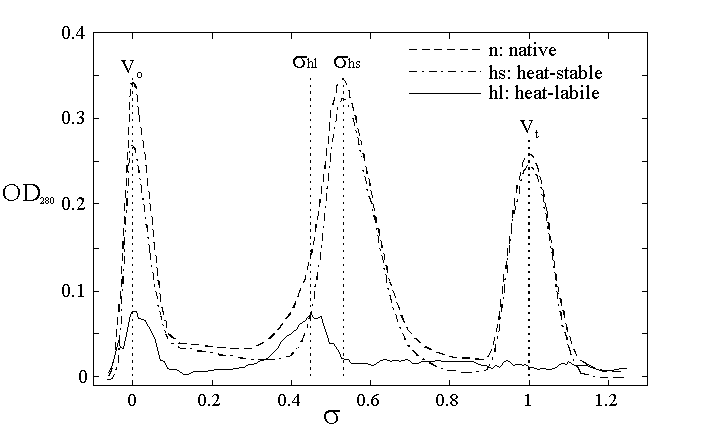
Fig. 2. Gel permeation chromatography of Winter Flounder egg yolk extract on a 1 meter BioGel A1.5 column. The optical density at 280 nm is plotted against the column partition coefficient, . Native extract (n) is compared to heat stable extract (hs) and their difference used to identify a heat-labile (hl) fraction of the native profile. (from Hartling et al., 1997). The identified peaks of hs- and hl-Lv have substantially different column partition coefficients, allowing their separation based on gel permeation alone. The void volume, Vo, is marked by blue dextran and the total volume, Vt, by the pthalate ion (Hartling et al., 1997).
While this approach produces in many cases a highly purified Lv product, our experience is that each species to be newly investigated presents possibilities of complications such as contaminating proteins or multiple forms of Lv. Further strategies for purification of fish Vg/Lvs include various adsorptive chromatographic techniques (Specker and Sulivan, 1994) such as DEAE- TEAE- or QAE- cellulose for acid proteins since Vg and Lv tend to have acidic groups on their surface. I prefer cellulose rather than Sephadex or acrylamide as a ligand-support for adsorbing Vg and Lv because in my experience gel penetration of the large proteins does not allow high binding capacities with the porous media in which the active ligands are found in the crevices of the support. Since Vg and Lv are lipoproteins, a hydrophobic column resin may also been useful in some species' Vg/Lvs.
In our purification of Winter Flounder Lv we were able to achieve a high degree of purification due to the relative thermal stability of native-Lv compared to other minor contaminants in an egg extract, including partially denatured Lv (Hartling, Pereira and Kunkel, 1997). We call this purification procedure fractional denaturation. In principle, the peptides of Lv or Vg that have been nicked by protease should be more vulnerable to thermal denaturation. No matter what is the actual thermal denaturation temperature for cod Lv or Vg, the proteolytically nicked protein should denature at a lower temperature and be easily removed by centrifugation. What remains after fractional denaturation is highly purified heat stable Lv, which can be further purified and characterized by gel permeation chromatography (hs-Lv in fig. 2). For Winter flounder that protein is highly stable and can be stored and used as a calibration standard for extended periods of time. We will try to extend this approach to purifying and using cod Lv.
Antigen characterization.
It is essential to characterize the macromolecular properties of both
Vg and Lv. In other vertebrates these macromolecules have
different subunit structure. Native cod Vg has been demonstrated
(Yao and Crim, 1996) to be a dimer of apparent Mr 485,000, including the
weight of adsorbed lipid (21%), attached phosphorous (2%) and two copies
of Lv and Pv still covalently bonded as a single peptide:
Native cod Lv, which has not yet been characterized, should include lipid plus a single or double copy of the lower Mr Lv peptide:
The potential substantial difference in molecular geometry means that Vg and Lv can give a different quantitative response in an immunologic assay (cf. The Marone saxatilis Vg/Lv assay of Kishida et al., 1992); but as long as we establish their relative molar equivalence and reactivities in the assay, we need only use one standard for the assay. My intuition is that the native Lv will be the most convenient standard for the Atlantic cod Vg/Lv ELISA.
The molecular weights and subunit compositions of the Vg and Lv will be determined by: SDS-PAGE to establish subunit purity and composition; gravimetric lipid extraction and analysis to determine the percent lipid content; and gel permeation to establish their native Stoke's radius. Since these properties have already been determined for Atlantic cod estrogen induced Vg, we will be performing a confirmation of its Stokes radius in our columns, which have been chosen such that a monomeric Lv will elute close to the middle of the column for maximal resolution, fig. 2. These properties will allow the determination of whether the Lv is monomeric or dimeric in its native form and will instruct us on what to expect for relative reactivities of Lv and Vg in the ELISA assay. It is not clear for instance whether the multiple forms of the huge amounts of Vg found in estradiol treated cod serum (Silversand et al., 1993; Yao and Crim, 1996) are all normal dimers or whether some proportion of abnormal monomers or tetramers are generated during the long accumulation of Vg in the serum. It is also assumed that the mucus and serum Vg would behave differently in the ELISA based on experience in striped bass (Kishida et al., 1992). In my mind, this may be due to differential quenching of the reaction by heterologous proteins or the presence of degraded forms of Vg in the mucus secretion (or serum). These alternatives must be examined since it has substantial signifigance to the accuracy of the ELISA. In this case the ability, through statistical design, to differentiate between accuracy and precision are tremendously important. No-matter how precise the assay, if differential quenching is destroying the accuracy of the ELISA, the results could be deceptive.
Monospecific Polyclonal Antiserum Production.
After purification of Lv (or Vg) the production of monospecific
polyclonal antiserum in New Zealand white rabbits is a routine procedure
(Kunkel & Pan, 1976; Kunkel, 1988; Hartling et al., 1997). Rabbits
rather than mice will be used to ensure a large supply of antiserum which
could be used for many years into the future.
Antiserum Characterization.
A polyclonal antiserum must be rigorously characterized after its production
before it can be declared monospecific. First, it must be shown to be of
sufficient titer and amount to achieve the long term objectives of the
research. Second, its specificity must be determined by showing that it
can measure the antigen in question (Vg or Lv) in a linear
monotonic fashion when the protein is in a relatively purified state. Third,
in situations where the antigen is mixed with many other proteins, a similar
monotonic response should be obtained (i.e. there should be no substantial
quenching of the response with heterologous proteins added to the homologous
protein).
Male serum will be used as a negative control and a quenching agent. It should not have any detectable Vg in it. When added to purified Lv it should not quench the ELISA response. A western blot of male serum should show no bands of immune staining protein. The purified Lv protein is the positive control and ELISA standard. A Western blot of fresh vitellogenic female serum should show a high molecular weight Vg peptide band of approximately 167 kDa (Yao and Crim, 1996). Any minor bands of immune precipitate should be lower molecular weight and should be stainable with antibody eluted from the 167 kDa band (Hartling et al., 1997). No substantial antibody reacting with the vitelline membrane proteins should be detected (Silversand et al., 1993). If a female Atlantic cod is mature and is vitellogenin and can be stripped of ovulated eggs, the eggs should show a processed lower Mr band corresponding to Lv peptide in SDS-PAGE western blot. The ovarian tissue may show various stages of processing of Vg peptide to the Lv and Pv peptides. In ovaries of winter flounder a dimeric form of Vg/Lv is found but by the time of ovulation all has been transformed into monomeric Lv (Hartling et al., 1997). Such a process could be expected in cod as well. This uncertainty in the form of Vg/Lv in the ovary may make it impossible to do a reliable quantitative assay of ovarian Lv.
Vg and Lv ELISA Assay.
An enzyme linked immunoassay (ELISA) for cod Vg and Lv
would use the monospecific polyclonal antiserum to measure the amount of
Vg or Lv in any extract or solution in question. We have
developed such an assay for Winter flounder Vg (Hartling, Pereira
and Kunkel, 1995, 1997; CMER project 92-07) as well as several other insect
proteins and have established criteria which need to be satisfied in order
to have a useful assay.
(1) A stable standard must be established. Aliquots of purified Vg or Lv need to be stored in a manner that allows them to serve as a standard among and between assays. Thus for example assays performed in 1996 must be comparable to assays performed in 1997 and so on. This requires that either an easily purified standard be available or that the standard once purified can be stored indefinitely. We will aim to provide one or the other of these alternative schemes. An easily purified standard needs to be characterized so that its concentration (in µM or mg per ml) can be easily estimated by using its OD280nm or a colorometric assay. It is already documented that purified Vg from Atlantic cod is unstable even with substantial precautions taken (Silversand et al., 1993).
(2) A routine of using standards with each ELISA will be established to predict the molarity of the measured protein.
(3) While the estimation of Vg or Lv will be based on a single standard, any differences in reactivity of Vg or Lv or any quenching of the reaction by other potentially conflicting proteins, such as heterologous protein or non-precipitating peptide antigen, must be established. We have substantial experience in this respect. Separate standard curves using both serum and mucusVg and Lv will be established and related to the primary standard (Lv) so that the proper molarity of each native protein can be estimated. The effects of natural quenching agents on each response will be investigated.
Ovarian Histology.
The histology of the Atlantic cod ovary has been studied (Morrison,
1987). While the ovarian stage, Ripening 2, when yolk droplets first
appear is described, its relationship to the annual cycle of estrogen secretion
and Vg secretion from the liver has not been established. We propose
to develop indices of ovarian follicle maturation (OMIs) which will enable
us to plot serum and mucus Vg titer vs various aspects of ovarian
status. Together with the standard gonadosomatic index (GSI) of gonadal
development, we suggest that a tissue level index of ovarian status may
provide additional information. The cod ovary can contain several age classes
of oocytes in its ovary, all the way from oögonia to hydrated eggs,
even in mature adults. Fecundity, no doubt, involves that fraction of the
available germ cells which rapidly mature in a given year. The behavior
of the current maturing cohort may be critical to determining the quality
of the resultant ripened eggs. In particular it is possible that the transition
from the "protoplasmic" growth to the "trophoblastic" growth stage can
be quantified. The OMIs will involve estimating the proportion of cross-sectional
area of the ovary that is devoted to various aspects of oocyte maturation:
Vg deposition (yolk droplet?), oocyte nuclei vs cytoplasm (protoplasmic
vs trophoblastic growth), hydrated oocytes, attretic follicles (Morrison,
1987). The measurement of attretic follicles might be of interest because
it could indicate how many batches of eggs a particular female had produced
during the current season prior to capture. This is significant because
the earliest egg batches of a female in the season are the largest (Kjesbu,
1989).
These attempts to estimate cytological stages of maturation will be based on applying Chalky grids (Locke and Collins, 1965) to sections of ovary, a quantitative morphological approach used by cytologists to determine the proportion of cells devoted to particular organelle compartments. In this approach random dots are projected onto images of tissue sections and the compartments the dots fall in are estimated statistically by using binomial theory to estimate proportions and associated error. The morphological structures that are incorporating Vg will be identified by immunofluorescent staining of ovarian sections. Attretic follicles, hydrated oocytes and other compartments will be identified by their light microscope morphology (Morrison, 1987). We have experience with this approach from our recent demonstrations of calmodulin localization in insect follicles (Zhang and Kunkel, 1994). If all the structures (including vitellogenic follicles) are morphologically distinct then the morphological study could proceed without each section being immunofluorescently stained. I suspect that such is the case. Our instructions for collecting and fixing ovarian tissue will initially be given such that immunofluorescence studies could be done.
We have the substantial microscope, video and computer image analysis capabilities to allow the Chalky grid evaluation of the ovarian tissue to be carried out. A Pentium based computer with Ethernet access to a large disc drive would be used as a work station to allow us to collect, store and retrieve the large numbers of tissue images which need to be analysed for our research.
Data Analysis.
Joe Kunkel is trained and proficient in biometrical techniques, having
spent a postdoctoral period in a biometry department and having devoted
a substantial part of his career to the subject of data analysis. He will
design experiments and analysis of the data such that error limits on measurements
and confidence level in conclusions can be quoted. When the ovarian maturity
index and ELISAs are being applied to samples during the second year, the
assays will be run double blind. The correlative data will be measured
by the NOAA personnel and withheld until the Amherst assays are finished.
The resultant multivariate data will be submitted to Analysis of Dispersion
and Discriminant Function Analysis (Rao, 1965) to answer several
questions. The PI has written and maintained software implementations of
these approaches over the course of the past 28 years. With Analysis of
Dispersion one can ask if there is any additional information about a particular
ovarian maturation index (OMIx) captured by the measure of Vgserum
that is not captured by Vgmucus an GSI (and vice versa).
With Discriminant Function Analysis we may be able to discover a simple
or minimally invasive predictor of OMIs. The future of this approach resides
in the ability of analytical data from the biochemical and cytological
lab combined with traditional measures, such as age-class and GSI, to be
used for predicting the reproductive status of a fish stock. This might
require future collection of data from other Atlantic cod stocks, such
as the Barents Sea stock which mature at seven to ten years, which might
be classified as 'healthy' stocks or under less pressure, to establish
a database which can be hypothetically thought of as a target of health
to which the Georges Bank stock might aspire.
This type of individual-oriented multivariate data collection would
be consistent with the development of a Markov chain model of the physiological
changes that take place in a population during annual cycles and processes
of maturation and aging of a population. This type of model would also
allow inclusion of the effects of harvesting on this process. It is a challenge
for us to apply our biochemical and cytological approaches to a problem
that might have major consequences to the health of ground fish stocks.
The projected products of this work can be categorized into four objectives which must allow for some latitude to allow for unforeseen eventualities:
1. A Purification scheme for cod Vg and Lv.
By three months into the first year a purification scheme for Atlantic
cod Lv will have been devised, (or we will have switched to using
estradiol induced Vg purification). Experience has taught that although
Vg and Lv are relatively easy to purify as a class, some
situations lead to more difficulty. As long as there is one immunologically
distinct Vg or Lv the purification depends on the relatively
dramatic difference in size and sometimes surface groups on the native
proteins. Whichever protein is able to be more highly purified will be
used in the immunization process. It is already known that estradiol induced
Vg from cod is heterogeneous. The protein of choice will be considered
the homologous antigen for the immunization and most likely would also
be the antigen of choice for the calibration procedure. The other protein
(as well as mucus Vg) need only be highly enriched (not highly purified)
in order for the ELISA assay to be validated in its measurement. With some
estimate of its purity, the other non-homologous antigens can also be calibrated
relative to the homologous antigen and tested for quenching effects. Usually
an estimate of purity can be obtained for a protein by doing a native PAGE
gel and estimating the proportion of the staining that falls in the main
peak of the semi-purified fraction.
Thus the purification scheme will provide an homologous antigen, a method for obtaining an accurate standard and hopefully a substantial amount of one of the proteins that we can store and use as a standard for several years. We have successfully stored Winter flounder Lv frozen in aliquots for three years. It is entirely possible that storage of Atlantic cod Vg or Lv can be done the same way. We are also familiar with alternate storage techniques to apply if the simple freezing scheme does not work, such as ammonium sulfate precipitation and storage at 4C or at -20C as 50% glycerol solutions. The purification and storage schemes adopted will be communicated as soon as possible with our NOAA colaborators and will also appear in our First Annual Report.
2. A Monospecific Polyclonal Antiserum against Atlantic cod Vg
or Lv.
As long as the antigen is a single protein, we aim to provide a pooled
stock of antiserum which could be used in the current studies with substantial
remaining for future studies. The antiserum will be collected for as long
as the rabbits are producing high titer specific antiserum. Each bleeding
will be processed to the crude gamma-globulin stage and tested for its
titer and specificity against the homologous antigen and the resultant
appropriate batches will be pooled in inverse proportion to their titer
to produce a pooled antiserum. The pooled antiserum will be aliquoted into
4 ml serum storage vials and made 1/3 saturated with ammonium sulfate for
long term storage at 4C. This method has been used by us to store antisera
for decades without substantial deterioration.
The antiserum and aliquots of standard protein will be made available to qualified researchers through the auspices of NOAA as long as the supply lasts.
3. A calibrated ELISA assay for Atlantic cod Vg and Lv.
This will include separate standard curves for serum and mucus Vg
and Lv. The homologous antigen will be used as a standard and the
limits of the assay will be determined. The threshold for a measurable
response will be measured at the low end of the scale. A linear response
range will be established. In addition the ability of male Atlantic cod
serum to quench the immune response will be tested at several Lv
and Vg concentrations. Small peptides derived from estradiol injected
off season cod (Silversand et al., 1993) will be produced, isolated and
their interference with the assay for native Vg and Lv will
be assessed.
Table 1. Hypothetical Multivariate Data Record structure for the study of Atlantic cod, Gadus morhua, female reproductive development. Each record refers to the properties of an individual. The record structure is composed of data field descriptors, followed by a thumbnail description of the field content. Details on some of the fields are elaborated in the text as well as key references (Morrison, 1987). All proposed OMIx traits are discernible at the light microscope level.
Data fields assigned or determined by NOAA collaborators:
ID# -an individual ID# for a particular fish caught at sea.
date -the date the specimen was caught and bled.
live-weight -the total weight as caught.
length -representing any standard measurements that characterize cod.
ovary-weight -one parameter of ovarian maturity and yearly vitellogenic cycle.
GSI -gonadal somatic index = (ovary weight)/(live weight -ovary weight).
age class -determined by measurements of otoliths.
egg diam. -average diameter of freshly ovulated or stripped eggs.
------------------------------------------------------------
Data fields determined by Kunkel laboratory:
OMI1 -index of ovarian maturity based on ovarian trait 1.
.
OMIh -index of ovarian maturity trait h, vesiculated ring oocytes.
OMIi -index of ovarian maturity trait i, protoplasmic oocytes.
OMIj -index of ovarian maturity trait j, trophoblastic oocytes.
OMIk -index of ovarian maturity trait k, hydrated oocytes.
OMIl -index of ovarian maturity trait l, attretic follicles.
.
OMIn -index of ovarian maturity based on ovarian trait n.
[Vg]mucus -titer of Vg in scraped mucus sample as a percent of total protein.
[Vg]serum -Vg titer in the serum.
Lv/egg -Lv content per egg (assayed on homogenates of 10 eggs).
V(Lv/egg) -variance of Lv content per egg (assayed on 10 separate eggs).
-------------------------------------------------------------
4. Correlation of serum Vg and egg Lv with age of female
and ovarian staging.
This portion of the project depends on the variety of samples that
our NOAA collaborators are able to provide us with and how far the supplies
budget can be stretched. It is somewhat open ended and could form the basis
for a students Masters thesis or Ph. D. dissertation. At a minimum we should
be able to correlate the general morphology of the ovary for different
age classes of Atlantic cod (Morrison, 1987) with the Vg titer in
their serum and mucus as well as establishing the average and variance
of Lv content for eggs derived from different age classes in the
Georges Bank cod stocks. Thus a data record for a particular female Atlantic
cod might be profiled in Table 1. The record structure for the actual data
collection will be agreed on in collaboration with our NOAA collaborators
by the tenth month of the first year. The selected data structure will
be included in our First Annual Report.
In the future, a similar table might be developed for the development of the Atlantic cod eleutheroembryo and larva (Morrison, 1995). Our experience with the flounder embryo suggests that a majority of Lv is not used for early embryonic development but is reserved for the critical stage which occurs between hatching and when feeding begins (Hartling and Kunkel, 1995). Such a table might provide valuable information on stressed population larval recruitment trends.
These multivariate data on individuals will aid in deciding whether young but mature Atlantic cod are physiologically capable of producing healthy eggs. Any pathology of a genetic or compensatory physiology may show up in the numbers being recorded. For instance, do early maturing females have abnormal levels of Vg in their serum when compared to the developmental state of their ovary? Is their a lack of concordance of the ability of their liver to produce Vg and the ovaries ability to clear it into developing oocytes? Are eggs from young females more highly variable in size and Lv content than those of more mature females? Analysis of trends in the multivariate data may allow a prediction if enough viable eggs can be produced to allow adequate recruitment to sustain the stock under reasonable harvesting pressures. With application of principles of multivariate analysis the combined information from NOAA biologists and the biochemical laboratory may provide answers that separately could not be reached.
A Second Annual Report will describe our activity during the year of data collection and describe the scope of our projected analysis of the data will be due at the end of the second year.
A description of the collected database, the database and an our preliminary analysis of the database will be submitted in a Final Report 6 months after the scheduled end of the two year project.
Cooperation with NOAA is absolutely essential for this project. Frank Almeida of the Woods Hole National Marine Fisheries Laboratory will arrange the collection of the necessary biological materials using their usual resources for collecting Atlantic cod on the Georges Bank. The following types of biological samples will be needed:
(1) Vitellogenic female serum and male serum and mucus. Serum will be obtained by clotting in the presence of protease inhibitors Aprotinin and PMSF. Some serum from fish injected with Aprotinin 30 min. prior to bleeding will also be requested in order to control for bleeding induced proteolysis of the Vg peptides. This is, in our experience, more a problem with estradiol induced Vg accumulation in immature or male fish. The female serum is for characterization and potential purification of Vg and should come from females predicted to have high natural Vg titer. The male serum should come from large adult males to provide a large volume. We need large volumes of this serum, both male and female, because it will be used in preparative amounts in developing the purification process and the larger the original batches the less we will incur complications from switching batches during the exploratory stage of purification. The various techniques for purifying Vg from serum are Vg-titer dependent (Silversand, 1993). The male serum will be used for potential adsorption of non-female specific antibodies from the crude antiserum and for quenching curves during the calibration procedure.
Large scale mucus collection from both vitellogenic females and males should be carried out with care to prevent degradation during collection and storage. We will devise a protocol which produces a protease inhibited, stable situation which will allow samples at sea to be transmitted to the lab for analysis. Tentatively large scale collection will involve scraping and transferal of the mucus to a saline containing protease inhibitors which will then be precipitated with 2/3 saturated ammonium sulfate and storage at 4C until transported to our lab. A method similar to this has been used by industry for decades to send enzymes through the mail. We have obtained specimens of biological fluids this way from field workers in Russia.
(2) Spawned eggs (unfertilized). Obtained by stripping gravid females and stored frozen aliquoted in vials. Initially we will want large samples of eggs (~4 ml per aliquot) from clearly mature females (2+ age classes) so that we can avoid any complications of quality of eggs and Lv from the 1st year class.
(3) Ovaries sampled and fixed for histological examination. The objective will be to obtain as wide a sample of ovarian tissue as possible to determine the range of ovarian maturation from several times during the year, covering several age classes. The light microscope tissue fixation routines of Morrison (1987) will be followed unless more stringent fixation for immunofluorescence is needed.
(4) Individually identified specimen records. Complete data records need to be obtained from individual fish for a multivariate information database. Thus the NOAA collaborators will need to collect the usual statistics on fish age and maturity which are normally used in addition to the mucus, serum and egg samples where possible to allow us to establish an Atlantic cod database of individual properties. We will provide the analyses of serum, mucus and egg Vg/Lv as far as possible as well as an ovarian maturity index.
(5) Immature or spent males and females for injection of estradiol-17ß. High titer Vg serum would be generated to provide a convenient supply of purified Vg, Vg derived peptides and vitelline peptides using the published purification techniques (Wiley et al., 1979; Silversand, 1993). This Vg source will be more important if the Lv purification is impossible or the amounts of enriched Vg attainable from normal vitellogenic females is insufficient to carry out calibration of the Vg assay.
At the conclusion of the proposed two year period we should be able
to evaluate whether further collaboration on an Atlantic cod database of
individual fish properties, such as OMIs, Vgserum , age-class
and GSI will be useful in evaluating the health of particular cod stocks
in the future or in answering questions on the relative health of separate
stocks which are not exhibiting the same pressures as the Georges Bank
stock. Such an evaluation will appear in our Final Report, six months after
the end of our two year project.
Goodwin, A. E., J. M. Grizzle, J. T. Bradley, and B. H. Estridge. 1992. Monoclonal antibody-based immunoassay of vitellogenin in the blood of male channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. 101B:441-446.
Hartling, R. C., J. J. Pereira, and J. G. Kunkel. 1995. Processing and degradation of lipovitellin during embryonic and larval development of the winter flounder (Pleuronectes americanus). FASEB J. 9:A81.
Hartling, R. C., and J. G. Kunkel. 1995. Proteolytic cleavage of yolk protein during flounder (Pleuronectes americanus ) development: characterization of lipovitellin from eggs and embryos. Mol. Biol. Cell 6:321a.
Hartling, R. C., J. J. Pereira, and J. G. Kunkel. 1997. Characterization of a heat-stable fraction of lipovitellin and development of an immunoassay for vitellogenin and yolk protein in winter flounder (Pleuronectes americanus). J. Exp. Zool. 278: 156-166.
Hyllner, S. J., and C. Haux. 1992. Immunochemical detection of the major vitelline envelope proteins in the plasma and oocytes of the maturing rainbow trout, Oncorhynchus mykiss. J. Endocrinol. 135:303-309.
Hyllner, S. J., D. O. Oppen-Berntsen, J. V. Helvik, B. T. Walther, and C. Haux. 1991. Oestradiol-17ß induces the major vitelline envelope proteins in both sexes in teleosts. J. Endocrinol. 131:229-236.
Kishida, M., T. R. Anderson, and J. L. Specker. 1992. Induction by ß-estradiol of vitellogenin in striped bass (Morone saxatilis): characterization and quantification in plasma and mucus. Gen. Comp. Endocrin. 88:29-39.
Kjesbu, O. S. 1989. The spawning activity of cod, Gadus morhua. J. Fish Biol. 34:195-206.
Lee, K. B. H., E. H. Lim, T. J. Lam, and J. L. Ding. 1992. Vitellogenin diversity in the perciformes. J. Exp. Zool. 264:100-106.
Locke, M., and J. V. Collins. 1965. The structure and formation of protein granules in the fat body of an insect. J. Cell Biol. 26: 857-885.
Methven, D. A., L. W. Crim, B. Norberg, J. A. Brown, G. P. Goff, and I. Huse. 1992. Seasonal reproduction and plasma levels of sex steroids and vitellogenin in Atlantic halibut (Hippoglossus hippoglossus). Can. J. Fish. Aquat. Sci. 49:754-759
Mommsen, T. P., and P. J. Walsh. 1988. Vitellogenesis and oocyte assembly. In: "Fish Physiology, Vol 11A" W. S. Hoar, and D. J. Randdall, Eds., Academic Press, San Diego, pp. 347-406.
Morrison, C. M. 1987. Histology of the Atlantic Cod, Gadus morhua: An Atlas. Part three, reproductive organs. Dept. Of Fisheries and Oceans, Ottawa, Ontario, Canada.
Morrison, C. M. 1995. Histology of the Atlantic Cod, Gadus morhua: An Atlas. Part four, the eleutheroembryo and larva. Dept. Of Fisheries and Oceans, Ottawa, Ontario, Canada.
Oppen-Berntsen, D. O., S. J. Hyllner, C. Haux, J. V. HeLvik, and B. T. Walther. 1992. Eggshell zona radiata-proteins from cod (Gadus morhua): extra-ovarian origin and induction by estradiol-17ß. Int. J. Dev. Biol. 36:247-254.
Rao. C. R. 1965. Linear Statistical Inference and Its Applications. John Wiley, New York, 522pp.
Silversand, C., S. J. Hyllner, and C. Haux. 1993. Isolation, immunochemical detection, and observations of the instability of vitellogenin from four teleosts. J. Exp. Zool. 267:587-597
Specker, J. L. And C. V. Sullivan. 1994. Vitellogenin in fishes: status and perspectives. In Perspectives in Comparative Endocrinology, National Research Council, Canada, p 304-315.
Trippel, E. A. 1995. Age at maturity as a stress indicator in fisheries. BioScience 45:759-771.
Wallace, R. A. 1985. Vitellogenesis and oocyte growth in nonmammalian vertebrates. In: "Developmental Biology, Vol. 1" L. W. Browder, Ed., Plenum Press, New York, pp. 127-177.
Wallace, R. A., and K. Selman. 1985. Major protein changes during vitellogenesis and maturation of Fundulus oocytes. Dev. Biol. 110:492-498.
Wiley, H. S., L. Opresko, and R. A. Wallace. 1979. New methods for purification of vertebrate vitellogenin. Anal. Biochem. 97:145-152.
Yao, Z., and L. W. Crim. 1996. A biochemical characterization of vitellogenins isolated from the marine fish ocean pout (Macrozoarces americanus L.), lumpfish (Cyclopterus lumpus) and Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. 113B:247-253
Zhang Y and J. G. Kunkel. 1994. Most egg calmodulin is a follicle cell contribution to the cytoplasm of the Blattella germanica oocyte. Developmental Biology 161:513-521.
8. Personnel Profile
Born: Oceanside, New York, August 17, 1942 AA# xxx-xxx-xxxx
Home: 74 Stony Hill Rd. University: Biology Department
Amherst MA 01002 University of Massachusetts
Home Phone: (413) 253-3391 Amherst MA 01003
Office/Lab Phone: (413) 545-0468 Email: joe@bio.umass.edu
Education:
Columbia College, New York, New York, Zoology A.B. 1964
Case-Western Reserve University, Cleveland, Ohio, Biology Ph.D. 1968
Dissertation: Control of Cockroach Development
Awards and Honors:
NSF Graduate Fellow, Biology, Case Western Reserve University, 1967-68; R.C.A. Scholar in Chemistry, Columbia College, 1961-62; Columbia University Scholar, Columbia College, 1960-64; New York State Regents Scholar, Columbia College, 1960-64; Bnai Brith Scholar, Columbia College, 1960
Positions and Professional Experience:
Sabbatical, National Vibrating Probe Facility, MBL, Woods Hole, MA 1993-94
Full Professor, University of Massachusetts at Amherst 1985-present
Adjunct Professor of Entomology, University of Massachusetts. 1985-present
Sabbatical with B. Lanzrein, Zoological Institute, U. of Berne, Switzerland, 1985-86
Member, Cell and Molecular Biology Program 1983-present
Associate Professor of Zoology, University of Massachusetts. 1976-85
Sabbatical with Alan C. Wilson, University of California at Berkeley 1977-78
Assistant Professor of Zoology, University of Massachusetts, Amherst 1970-76
Lecturer in Biology, Yale University 1969-70
NIH Postdoctoral with Gerry R Wyatt, Yale University 1968-70
Postdoctoral in Biometry, Case Western Reserve University Medical School 1968
Graduate Research Assistant, Biology Department, CWRU, Cleveland 1964-68
Research Assistant to Arthur W. Pollister, Columbia University, NY 1963-64
Research Assistant to Francis J. Ryan, Columbia University, NY 1963
Postdoctoral and Research Associates:
Rolf Koenig, Postdoctoral Research Associate, 1985-86
Elizabeth S. Bowdan, Senior Research Associate, 1986-1993.
PhD Students: Raymond Duhamel PhD '79, Don Wojchowski PhD '84, Sharon Karpells PhD '88, Yujun Zhang PhD '92, Rachel Dompenciel PhD '93, Anand Iyengar '96, Ruth Hartling, Ellen Faszewski
Kunkel JG and JH Nordin. 1985. Yolk Proteins. in Comprehensive Insect Physiology, Biochemistry and Pharmacology. Chapter 4, Vol.I, eds. GA Kerkut and LI Gilbert, Pergamon Press, pp 83-111.
Kunkel JG. 1986. Dorsoventral currents are associated with vitellogenesis in cockroach ovarioles. in Ionic Currents In Development ed. R Nuccitelli, Alan R Liss Publ., pp 165-172.
Kunkel JG, R Koenig, H Kindle and B Lanzrein. 1986. The role of ions in vitellogenesis and patterning in insect oocytes. Advances in Invertebrate Reproduction 4: 101-108.
Kunkel JG (1988) Analytical Immunological Techniques. Chapter I in Immunological Techniques: Arthropods. Edited by LI Gilbert. Springer Verlag, pp 1-41.
Kunkel JG. 1991. Models of pattern formation in insect oocytes. In Vivo 5: 443-456.
Duhamel RC and JG Kunkel. 1983. Cockroach larval-specific protein (LSP), a tyrosine-rich serum protein. J. Biol.Chem. 258: 14461-14465.
Wojchowski DM, PA Parsons, JH Nordin and JG Kunkel. 1986. Processing of Provitellogenin in Insect Fat Body: a role for High-Mannose Oligosaccharide. Dev. Biol. 116: 422-430.
Koenig R, JH Nordin, CH Gochoco and JG Kunkel. 1988 Studies on ligand recognition by vitellogenin receptors in follicle membrane preparations of the German cockroach Blattella germanica. Insect Biochem. 18, 395-404.
Kunkel JG and E Bowdan. 1989. Modeling currents about vitellogenic oocytes of the cockroach, Blattella germanica. Biol. Bull.176(S): 96-102.
Anderson M & JG Kunkel. 1990. Cleaning insect oocytes by dissection and enzyme treatment. Tissue & Cell 22: 349-358.
Bowdan E & JG Kunkel. 1990. Patterns of ionic currents around the developing oocyte of the German cockroach, Blattella germanica. Developmental Biology 137: 266-275.
Kindle H, B Lanzrein and JG Kunkel. 1990. The effect of ions, ion channel blockers, and ionophores on uptake of vitellogenin into cockroach follicles. Developmental Biology 142: 386-391.
Siegel E, R Baur, JG Kunkel, H Kindle, and B Lanzrein. 1990. Demonstration of a voltage dependent calcium current in follicles of the cockroach, Nauphoeta cinerea. Invert. Reprod. Devel. 18: 159-164.
Zhang Y and JG Kunkel. 1992. High abundance calmodulin from Blattella germanica eggs binds to vitellin subunits but disappears during vitellin utilization. Insect Biochem. Molec. Biol. 22: 293-304.
Zhang Y and JG Kunkel. 1992. Program of F-actin in the follicular epithelium during oogenesis of the German cockroach, Blattella germanica. Tissue & Cell 24: 905-917.
Zhang Y and JG Kunkel. 1994. Most egg calmodulin is a follicle cell contribution to the cytoplasm of the Blattella germanica oocyte. Developmental Biology 161:513-521.
Anderson M, E Bowdan and JG Kunkel. 1994. Comparison of defolliculated oocytes and intact follicles of the cockroach using the vibrating probe to record steady currents. Developmental Biology 162:111-122.
Bowdan E and JG Kunkel. 1994. Ionic components of dorsal and ventral currents in vitellogenic follicles of the cockroach, Blattella germanica. J. Insect Physiol. 40:323-331.
Kunkel JG and PJS Smith. 1994. Three-dimensional calibration of the non-invasive ion probe (NVPi) of steady ionic currents. Biol. Bull.
Kunkel JG and E Faszewski. 1995. Pattern of potassium ion and proton currents in the ovariole of the cockroach, Periplaneta americana, indicates future embryonic polarity. Biol. Bull. 189:197-198.
Iyengar AR, and JG Kunkel. 1995. Follicle cell calmodulin in Blattella germanica: Transcript accumulation during vitellogenesis is regulated by juvenile hormone. Developmental Biology 170: 314-320.
Kunkel JG and EE Faszewski. 1995. Pattern of potassium ion and proton currents in the ovariole of the cockroach, Periplaneta americana, indicates future embryonic polarity. Biol. Bull. 189:197-198.
Hartling, RC, JJ Pereira, and JG Kunkel. 1997. Characterization of a heat-stable fraction of lipovitellin and development of an immunoassay for vitellogenin and yolk protein in winter flounder (Pleuronectes americanus). J. Exp. Zool. 278: 156-166.