Use of Morphometrics and Biochemical Assays to
Study the Development of Larval Tautog.
Proposal response to CMER NOAA/NMFS RESEARCH TOPICS - 1998:
19. Effect of Dietary Fatty Acid and Amino Acid Composition on the Growth Rate and Body Composition of Larval Tautog (Tautoga onitis) and on the Reproductive Success of Adult Tautog
(contact: Dean Perry, 203-579-7030, and Laurel Ramseyer, 203-579-7022, Milford Laboratory)
Joseph Kunkel and Joseph Zydlewski
Status of research during March 1, 2000 through May. 31, 2000, on the effect of PUFAs on the growth and development of the larval and juvenile tautog (Tautoga onitis).
I. Lipid protein ratios
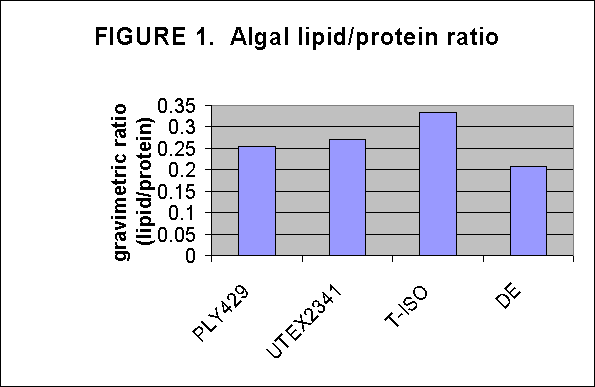
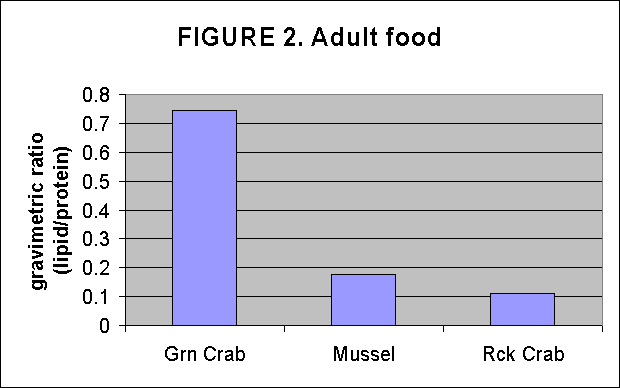
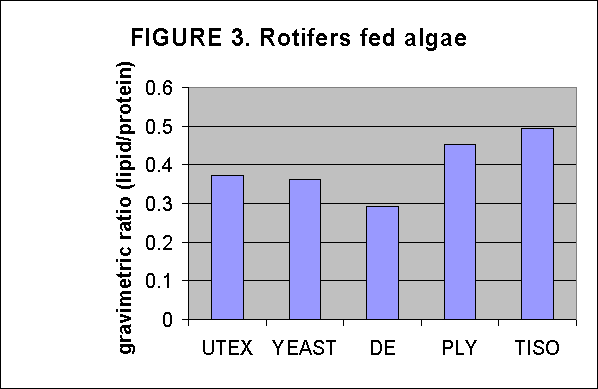
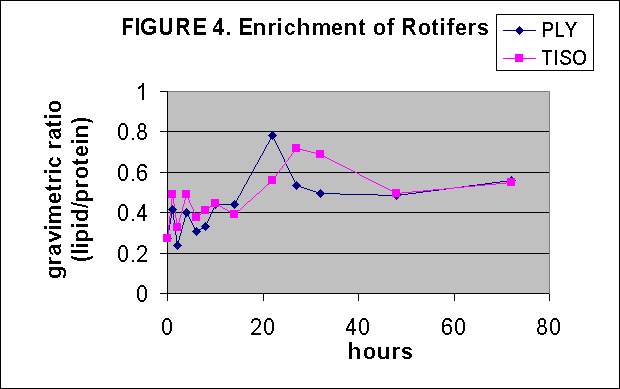
Crude lipid/protein ratios were determined on Tautog aquaculture food resources by determining lipid and residual protein content gravimetricly after chloroform/MeOH lipid extraction. The resultant data indicate that the alga strain T-iso has the greatest lipid content among the algae, Fig. 1. Green crabs are the best lipid source among the adult food items tested, Fig. 2. T-iso followed by Ply are the best soure of lipids for overnight enrichment of rotifers, Fig. 3. In the time series of rotifer enrichment it appears that 24 hours of enrichment achieves the highest lipid/protein ratios in the rotifers, approaching 0.7-0.8:1 by 24 hours after which the ratio declines, Fig 4.




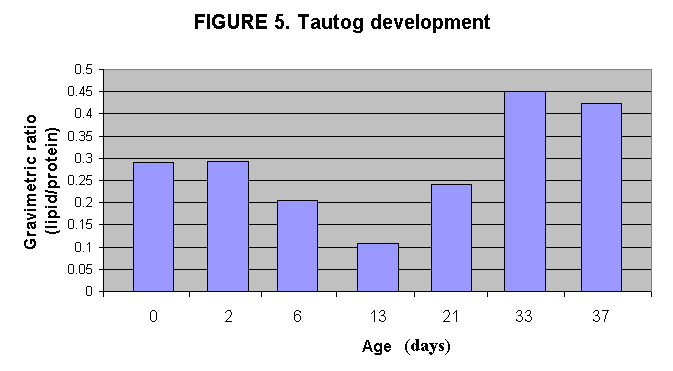
II. Lipid content during tautog development in aquaculture
Tautog eggs (0 days), larvae (2 - 21days after hatching) and juveniles
(33 and 37 days after hatching) sampled from the aquaculture facility at
NMF, Milford and submitted to lipid analysis. The larvae at 21 days
has resumed lipid accumulation, presumably from eating rotifers.
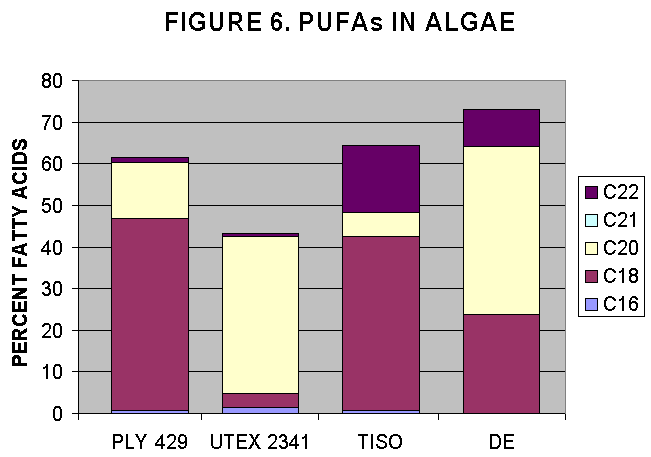
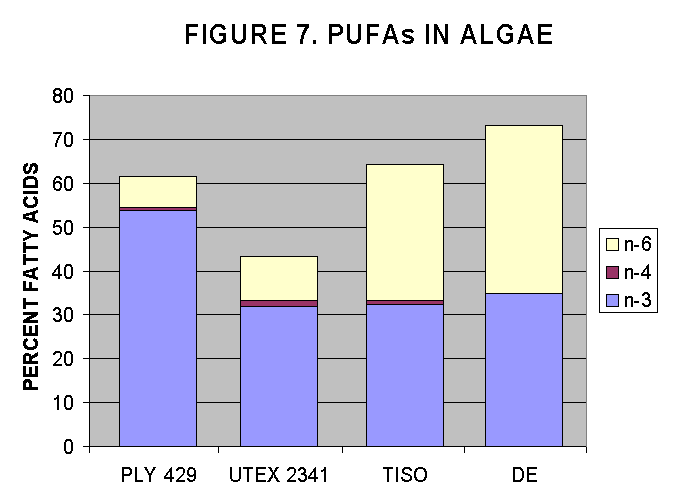
Algal strains were harvested from growing cultures at NMF, Milford lab, and were analysed for their FA content and then the PUFAs were estimated as a percentage of total FA.
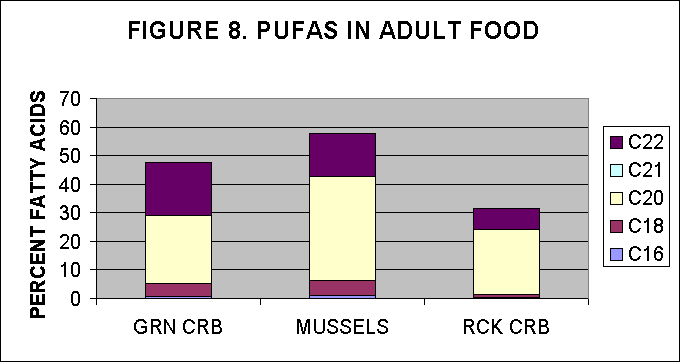
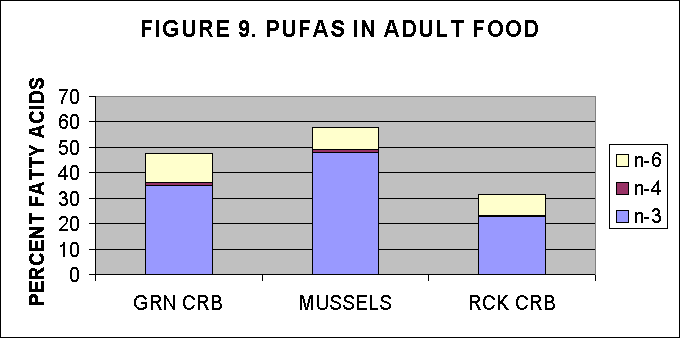
IV. PUFA content in adult food items
Adult food items were provided by NMF, Milford lab, and were analysed
for their FA content and then the PUFAs were estimated as a percentage
of total FA.
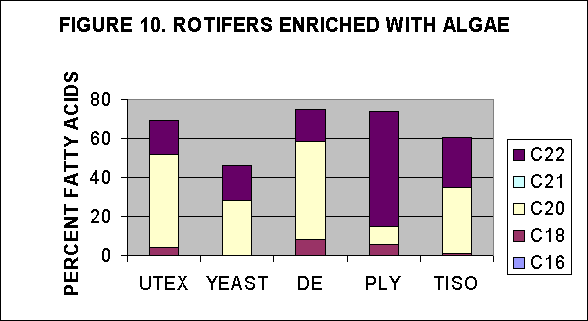
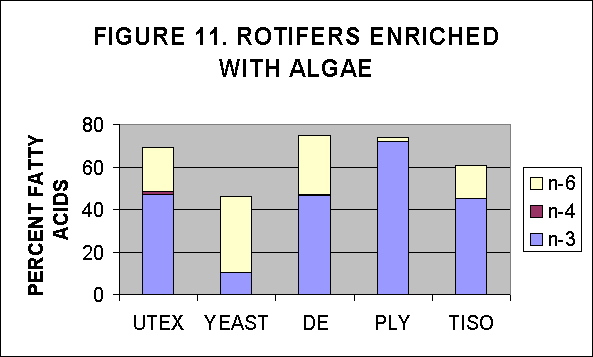
V. PUFA content in rotifers enriched with algal strains overnight
Rotifer cultures enriched with algae overnight were provided by NMF,
Milford lab, and were analysed for their FA content and then the PUFAs
were estimated as a percentage of total FA. Two enrichment experiments
were analyzed. In the first rotifers were enriched overnight (18
hours) and concentrated using a seive plus centrifugation of the seive
contents. This was a one time point analysis which resulted in one
estimate of lipid for each algal strain, Figs. 10 and 11. A second
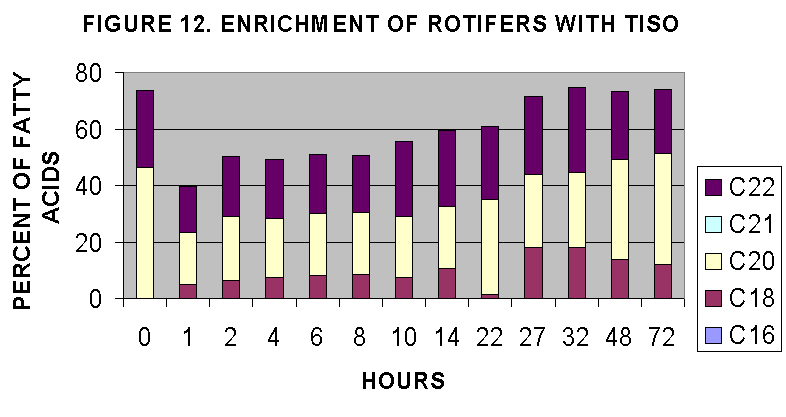
experiment concentrated on T-iso and Ply in a time course of enrichment.
This time course was done in paralell wuith the two algal strains and the
initial 8 hours of sampling was done at NMF, Milford Lab and the cultures
transported to UMass Amherst and subsequent sampling continued there.
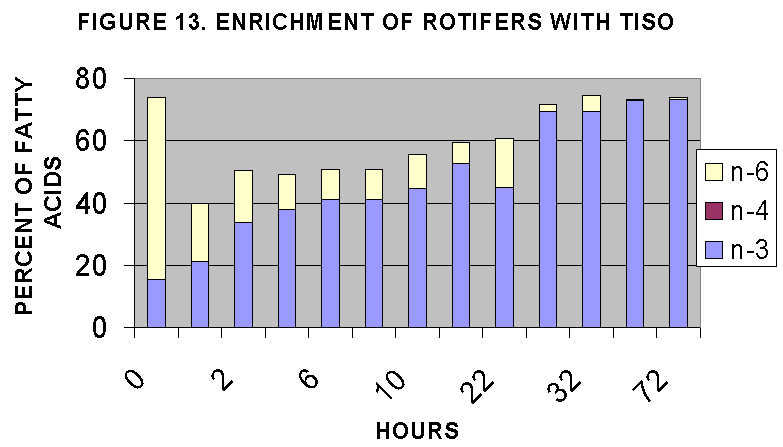
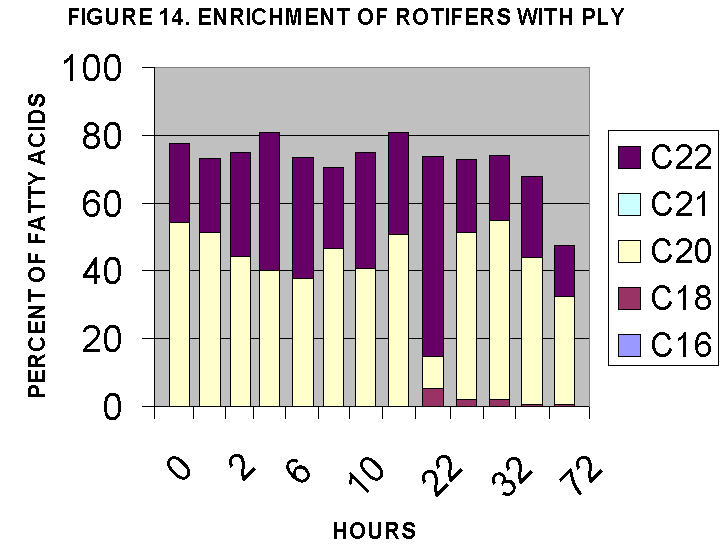
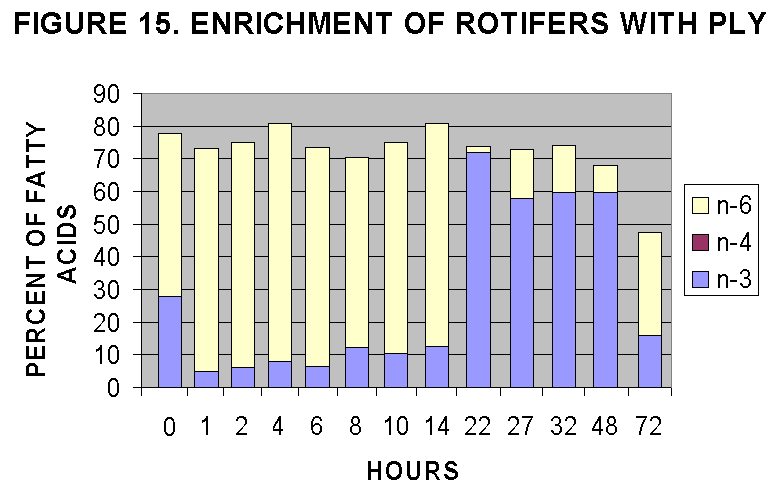
The two time courses are illustrated in Figs. 12-15. The time courses
suggest that maximal rotifer enrichment would be achieved at 20-24 hours
based on lipid/protein ratios (Fig. 4) and C18-C22 enrichment (Fig. 12,
14) and n-3,4,6 content (Fig. 13, 15). In general it seems that T-iso
VI. PUFA transfer to larvae and juveniles
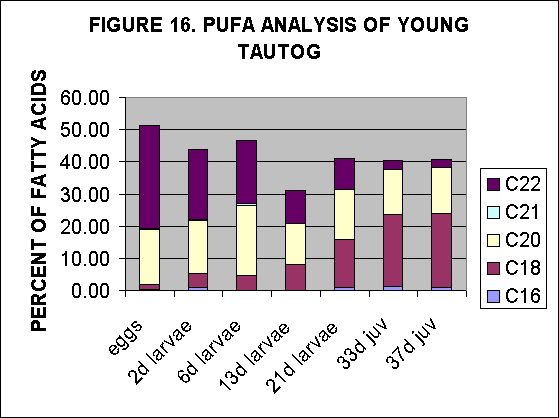
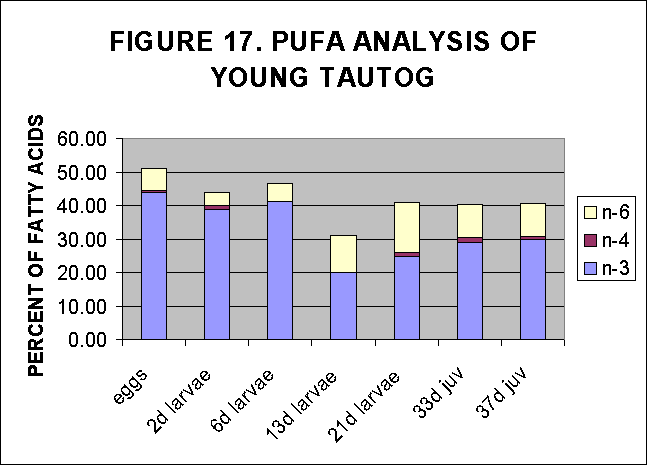
The 21day larvae and 33 and 37 day juveniles of aquaculture reared tautogs
(and perhaps 13 day larvae) demonstrate increased evidence of PUFA content
of their FA fraction as a result of feeding by algal enriched rotifers,
Fig. 16, 17.
VII. Conclusions
Since the tautogs were not on a specific diet regimen of one type of algal enriched rotifer, it is not possible to suggest which enriched rotifers would have resulted in better growth of the tautogs. However it was clear from analysis of the algae and the algal enriched rotifers that there were clear differences in the PUFA content of different algal strains and rotifers enriched by them, and that the appropriate timing of enrichment could produce rotifers of substantially higher PUFA content.
VIII. Active Personnel during this reporting period.
1. Joe Kunkel, PI. Morphometric analysis and protein purification
and analysis.
2. Joe Zydlewski, Postdoctoral Associate. Lipid analysis
and immunologic assay.
3. Ray Moniz, Undergraduate. Senior undergraduate student
assisting Joe Zydlewski in HPLC.
4. Rahul Sharma, Undergraduate. Graduate technician assisting
Joe Kunkel in immunology.
5. Jeff Xu. Graduate student working on image analysis.
Respectfully submitted,
Joseph G. Kunkel
7/7/2000