Experimentation with the carbonate LIX (IE-310 from World Precision) is still in development. Initial studies once thought this cocktail measured HCO3- but now it is now known to be CO32- selective. The measurement of this ion is very difficult due to the constant shifting in equilibration of CO32- and HCO3- ions. We have constructed carbonate electrodes according to Chesler et al. (Table) and have utilized a dual micromanipulator (holding both proton and carbonate electrodes) to determine the electrode slope.
The proton and carbonate concentrations of three low calcium Holtfreter's
solutions at varying pHs (7.3, 7.8, and 8.3) were measured. By calculating
the actual [H+] from the values obtained from the H+
electrode and dividing it by the bicarbonate dissociation constant of 6x10-11
(MacInnes and Belcher, 1935), it was possible to calculate the ratio
of HCO3- to CO32-.
The amount of carbonate in the initial bathing solution (3.38mM) was then
divided by this ratio to reveal the amount of carbonate (and bicarbonate)
that was actually present in the solution at that particular pH.
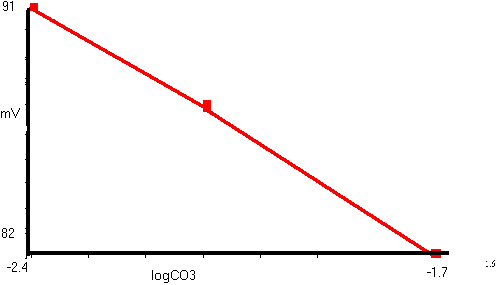
The logs of these carbonate concentrations were then used to calculate
the electrode slope of 14.3mV/decade change as viewed below:

Although this experimental slope is less than the theoretical 29.5mV for divalent ions, previous studies have also recorded lower values in the presence of Cl- and HCO3- ions (Chesler et al, 1994; Wietasch and Kraig, 1991).