JENSEN LAB
Research
Our research goal is to identify and define the cellular and molecular mechanisms involved in generating and maintaining the functional morphology of vertebrate photoreceptors. We use zebrafish as our primary model organism for this research.
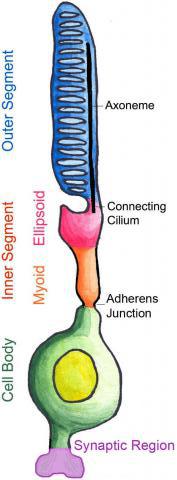
Vertebrate photoreceptors are specialized light sensing neurons that have a unique cellular morphology with four functional compartments:
Outer segment - highly modified cilium, packed with stacks of intramembranous discs containing the photon-capturing opsin proteins
Inner segment - major site of protein and lipid synthesis
Cell body - transcriptional regulation in the nucleus
Synaptic region - site of neurotransmitter release
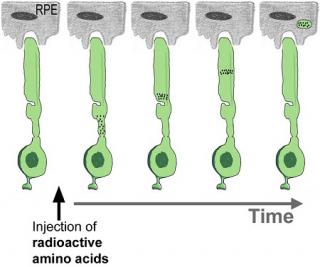
Decades ago, pioneering work by Young and colleagues (Young, 1967; Young and Droz, 1968;
Young and Bok, 1969; Young, 1971) first demonstrated, using injection of radioactive amino acids,
that rod outer segments are renewed continually.
Radioautography Method
The classical radioautography method of measuring renewal takes a long time ( months of exposure ) and is tedious. As a consequence, experiments using this method have been used rarely in recent years. Thus, little is known about the molecular mechanisms controlling outer segment renewal
Fluorescence Microscopy Method
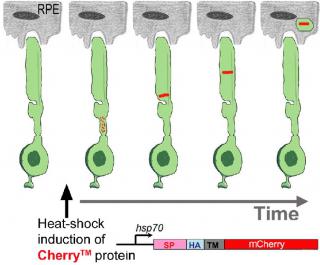
To identify molecular mechanisms of rod outer segment renewal, we developed a transgenic zebrafish, Tg(hsp70:HA-mCherryTM), that allows us to rapidly measure the kinetics of rod outer segment renewal (Willoughby and Jensen, 2012) using confocal fluorescence microscopy.
The classical radioautography method of measuring renewal takes a long time (months of exposure) and is tedious. As a consequence, experiments using this method have been used rarely in recent years. Thus, little is known about the molecular mechanisms controlling outer segment renewal
To identify molecular mechanisms of rod outer segment renewal, we developed a transgenic zebrafish, Tg(hsp70:HA-mCherryTM), that allows us to rapidly measure the kinetics of rod outer segment renewal (Willoughby and Jensen, 2012) using confocal fluorescence microscopy.
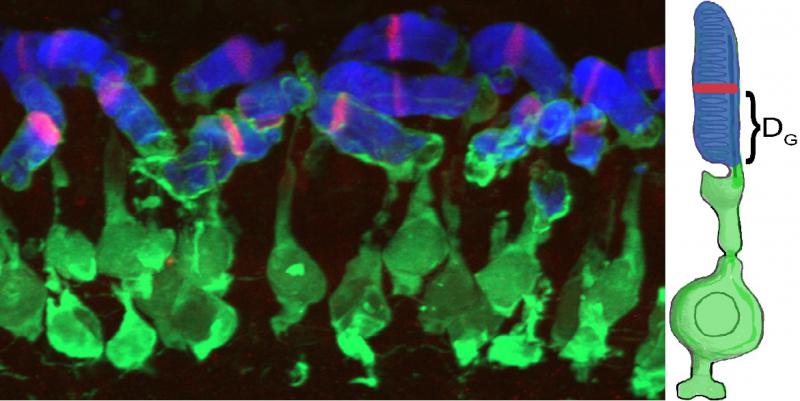
DG: using this measurement we can calculate growth rates
In some experiments, using Tg(hsp70:HA-mCherryTM), we can also measure shedding rates.
Basic questions about outer segment renewal remain:
• What is the growth molecular machinery that adds new material at the base of outer segment
• How do photoreceptors ‘know’ how much material to add daily?
• Is growth mostly constitutive or is it tightly regulated?
• What is the shedding molecular machinery that daily sheds chunks of distal outer segments?
• Is there an outer segment length set-point? If so, what molecules determine the set-point?
• Are the processes of growth and shedding coordinately regulated?
• Does deregulation of the renewal process contribute to retinal disease?
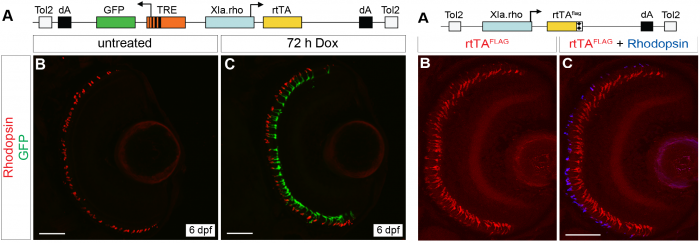
We developed two TetOn systems to temporally control transgene expression in rod photoreceptors
Because it is possible that some of the genes involved in outer segment renewal may also be crucial for initial outer segment formation, we developed two transgenic lines where transgene expression can be controlled by treatment with doxycycline (Campbell et al., 2012), so we can manipulate transgene expression after the outer segment has formed.