






Research interests and methods
Growth AnisotropySome Preliminaries Growth anisotropy describes the condition when growth rates are not equal in all directions. In contrast, when growth rates are the same rate in all directions, growth is isotropic. Anisotropy is a hallmark of plant growth. Almost without exception, cells grow faster in one direction than in another. When one direction has the fastest growth, it can be shown axiomatically that the direction with the slowest rate is orthogonal, and the ratio of maximal to minimal rate can be used to quantify the anisotropy of expansion. Another thing to keep in mind is that cell shapes are also anisotropic, being larger in one direction than in another, but the anisotropy of cell shape cannot in general be used to infer the anisotropy of expansion rate. This is because cell shape results not only from expansion but also from the placement of cross walls.. The present state of the art The current understanding of how cells grow anisotropically was laid in the 1960's by Paul Green, RD Preston , and others. The paradigm is that growth anisotropy is controlled by the deposition in the cell wall of cellulose microfibrils aligned on average perpendicular to the direction of maximal expansion rate. The parallel microfibrils set up a mechanical anisotropy in the cell wall that facilitates yielding (i. e. expansion) perpendicular to the long axis of the microfibrils and hinders yielding parallel to the microfibrils. In turn, the deposition of parallel microfibrils is accounted for by the orientation of cortical microtubules, which are customarily found to be parallel to the microfibrils. Trouble in paradigms The above paradigm falls short in a number of areas. First it ignores the degree of anisotropy. That is, during anisotropic growth, the ratio of maximal to minimal expansion rates may have a value anywhere from above 1 (maximal growth rate just exceeds minimal) to infinity (minimal growth rate equals zero) but there is currently no explanation for how this quantitative regulation is achieved. Second, it is not known how the microtubules influence the cellulose. Models have been proposed over the years but none have achieved more than hypothetical status. What's more, evidence is accumulating that calls into question the idea that microtubules orient microfibrils at all. It is possible that their effect on anisotropy could be mediated through some other means, as yet un-discovered. Third and finally, there is no idea whatsoever how the microtubules become oriented. Microtubule orientation typically reflects organ polarity but it is difficult to imagine how the known mediators of organ polarity, such as gradients in hormones, can specify an angular orientation within a cell. |
||
Experimental approaches I use roots as experimental material. Roots grow fast, are roughly radially symmetric and endowed by evolution with the ability to take things up from the medium, a property of considerable convenience to the experimenter. We have developed an in vitro system using maize roots that allows us access to the cortical array and underlying microfibrils. We have also isolated a series of root morphology mutants in arabidopsis that lets us work backwards to the proteins involved in controlling organ shape. Microtubules in vitro We have developed a new in vitro assay for characterizing the behavior of cortical microtubules. We cut sections of maize roots on a Vibratome and although most of the cytoplasm is lost from the cells, many cortical microtubules remain. The sections can be incubated as desired and then fixed and examined with either indirect immunofluorescence microscopy or FE-SEM. |
 |
|
 |
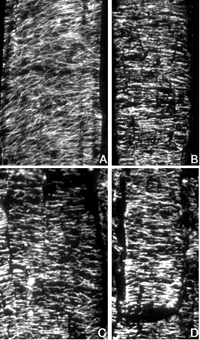
Above: Indirect immunofluorescence micrographs of microtubules in maize roots. Sections were cut of living, un-fixed roots, and fixed either immediately (A), or after 10 min (B), 20 min (C), or 30 min (D) incubation in buffer. Following fixation, microtubules were processed for imaging as described in Liang et al. 1996 Protoplasma 190: 8-15. Note the stability of most of the microtubules to incubation in buffer. Image courtesy of Gwo-Wei Tian and T. I. Baskin. |
|
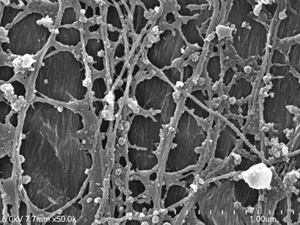
| Left: FESEM image of the cell cortex of a maize root. Microtubules are seen overlying scraps of plasma membrane, with the cell wall visible underneath where the plasma membrane has been lost. Note also the presence of polygonal patches typical of clathrin coated pits. | ||